Exclusive — Shocking Data Suggests Abortion Pill Complications 22 Times Higher Than Previously Reported: ‘FDA Must Reinstate Stronger Safeguards’
While the abortion pill is touted as “safe and effective,” shocking new data suggests complications are 22 times higher than previously reported.
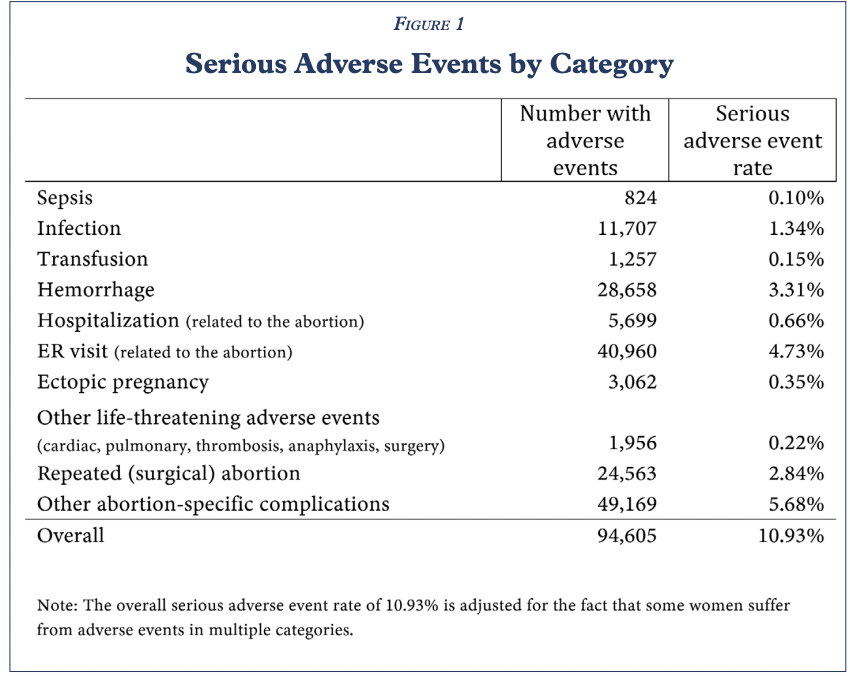
A study from the Ethics and Public Policy Center released on Monday found that 10.93 percent of women who had mifepristone abortions — the first drug used in a two-drug medication abortion regimen — experienced severe complications including sepsis, infection, hemorrhaging, or another serious adverse event within 45 days following the abortion. This percentage is significantly higher than the less than 0.5 percent in clinical trials reported on the FDA-approved drug label.
The study is the “largest-known study of the abortion pill,” according to the authors, Ethics and Public Policy Center President Ryan T. Anderson and Director of Data Analysis and Fellow Jamie Bryan Hall. The study is based on analysis of data from an all-payer insurance claims database that includes 865,727 prescribed mifepristone abortions from 2017 to 2023 — meaning the results of the study are based on real-world incidents. The data was studied by a team of data scientists, analysts, and engineers, and a clinical team of board-certified obstetricians and gynecologists assisted, they wrote.
“Contrary to what some may claim, the abortion pill is not like Tylenol. We find that one out of every ten women who takes the abortion pill will suffer from a serious adverse event, like hemorrhage or infection, soon afterward. A third to a half of these women will then go to the ER or even be hospitalized as a result,” Anderson and Hall told Breitbart News. Anderson is a Princeton graduate with a PhD in political science from Notre Dame, and Hall holds a bachelor’s and master’s degree from Harvard in applied math and statistics.
“This is unacceptable. We can be confident in our results because we’re using insurance claims data from more than 865,000 cases to track the real-world experiences of the women who have been harmed by the abortion pill,” they said.
In comparison, the current FDA-approved drug label for mifepristone is based on the results of ten clinical trials with a total of 30,966 participants, where less than 0.5 percent experienced serious adverse events. The current drug label also includes data from as early as 1983, according to the study.
By looking through insurance data and classifying cases of medication abortions through various medical coding, researchers identified a total of 865,727 mifepristone abortions for a total of 692,873 women, including 566,446 women who had one such abortion and 126,427 women who had multiple abortions.
From those incidents, researchers found a total of 94,605 adverse events related to medication abortions. The number includes:
“Simply stated, mifepristone, as used in real-world conditions, is not ‘safe and effective.’ Our real-world post-market observational study of mifepristone is, to our knowledge, the most comprehensive study of chemical abortion safety ever conducted in the U.S.,” the authors alleged.
The findings come after nearly a decade of Democrat presidential administrations deregulating the drug since its initial approval by the Clinton administration’s FDA in 2000.
Abortion Pill Approval and Deregulation
In a medication abortion, mifepristone — also called by the brand name Mifeprex created by Danco Laboratories — blocks the action of progesterone, which the mother’s body produces to nourish the pregnancy. When progesterone is blocked, the lining of the mother’s uterus deteriorates, and blood and nourishment are cut off to the developing baby, who then dies inside the mother’s womb. The drug misoprostol (also called Cytotec) then causes contractions and bleeding to expel the baby from the mother’s uterus.
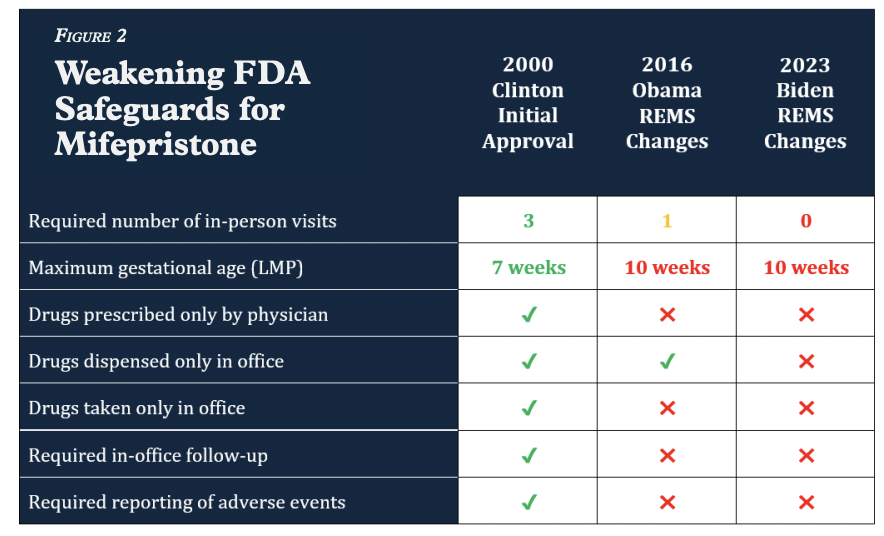
Mifepristone was fast-tracked for approval in 2000 during the Clinton administration under a process that required unwanted pregnancy to be classified as a “serious or life-threatening illness” and the drug had to be proved more effective than surgical abortion, according to the report.
The original FDA-approved drug label for Mifeprex from September of 2000 said the drug should be used through approximately 7 weeks of pregnancy. Its use also required “several modest safeguards for women’s health,” the study notes. Safeguards included:
- Three office visits by the patient
- A prescription given only by physicians who have read and understand prescribing information
- Administration of the drug in a clinic, medical office, or hospital, by or under the supervision of a physician able to assess the gestational age of an embryo and to diagnose ectopic pregnancies
- The presence of a physician who is able to provide surgical intervention in cases of incomplete abortion or severe bleeding, or plans for such care through others
- Patient access to medical facilities equipped to provide blood transfusions and resuscitation, if necessary.
But these safeguards have been chipped away over the past two decades since the drug’s approval.
Following actions from the Obama and Biden administrations, the drug’s current Risk Evaluation and Mitigation Strategy (REMS), which has been in effect since 2023 allows women to obtain mifepristone with one telehealth visit with any approved healthcare provider (not necessarily a physician), allows women to self-administer the drugs obtained from a mail-order pharmacy, and allows women to take the drugs up to ten weeks gestation instead of seven weeks.
The FDA also stopped requiring prescribers to report serious adverse events other than deaths in 2016.
Pro-abortion groups like Planned Parenthood and The American College of Obstetricians and Gynecologists (ACOG) have contended scrapping these regulations are necessary in order to increase access to abortions, especially in wake of the Supreme Court’s Dobbs decision overturning Roe v. Wade. Roe had created the constitutional right to abortion for 50 years before the high court released its Dobbs decision in 2022, sending the issue back to states and their elected representatives.
The American Association of Pro-Life OBGYNs (AAPLOG) counters that relaxing regulations around mifepristone puts women at risk. Specifically, they warn a lack of in-person evaluation could put women at risk of having undiagnosed ectopic pregnancies or miscalculating how far along they are.
“The abortion pill can cause an ectopic pregnancy to burst and put the woman’s life in danger. Yet we find that roughly one in 300 women who takes the pill in the real world is diagnosed with an ectopic pregnancy after she has already taken the pill,” the study’s authors told Breitbart News. “The FDA must reinstate stronger safeguards to prevent this from happening.”
“The further along the woman is in her pregnancy, the larger and more developed her unborn child is, and the greater the risk of complications from the abortion pill, such as retained fetal parts,” they continued. “By tightening gestational age limits and requiring the doctor to accurately assess gestational age before prescribing the pill, the FDA can reduce the risk of harm to the woman.”
The Abortion Pill Harms Women
When Breitbart News asked the study’s authors how the number of serious adverse events they recorded for mifepristone compares to other FDA-approved drugs, they explained that “a lot depends on what underlying condition the drug is intended to treat.”
“Drugs for moderate to severe conditions that do have higher serious adverse event rates are almost always prescribed because there are no alternatives. But even then, we also require stricter protocols and safety measures, and require that they be administered under a physician’s care,” they replied.
“We accept higher risks in cancer drugs than in Tylenol, for example. Advocates frequently claim that the abortion pill is just as safe as Tylenol. But 1 in 10 women taking the abortion pill experience a serious adverse event, whereas it’s in the range of 1 in 10,000, conservatively speaking, for Tylenol when taken as directed,” they continued. “And unlike other drugs, we no longer require the safety protocols and a physician’s care—that were originally required—because the Obama and Biden FDAs removed them.”
Danco Laboratories says on its website that “although cramping and bleeding are an expected part of ending a pregnancy, rarely, serious and potentially life-threatening bleeding, infections, or other problems can occur following a miscarriage, surgical abortion, medical abortion, or childbirth.”
“Seeking medical attention as soon as possible is needed in these circumstances. Serious infection has resulted in death in a very small number of cases. There is no information that use of Mifeprex and misoprostol caused these deaths. If you have any questions, concerns, or problems, or if you are worried about any side effects or symptoms, you should contact your healthcare provider,” the website states.
The Medication Abortion Landscape
The study’s findings carry significant weight, given that medication abortions have become the most common method of abortion in the U.S. after the Supreme Court overturned Roe v. Wade.
In 2023, medication abortions accounted for 63 percent of all abortions within the formal U.S. healthcare system — meaning an estimated 642,700 unborn babies died in medication abortions, according to the pro-abortion Guttmacher Institute. The percentage was up from an estimated 53 percent in 2020 and 39 percent in 2017. The report did not account for abortion pills obtained through underground national and international networks, including those that send pills to women in states with abortion restrictions.
And since Roe was overturned, the already politically divisive issue of abortion has gone radioactive and has been used as a linchpin in virtually every subsequent state and national election.
One such example is the death of Amber Nicole Thurman, a Georgia mother who died of sepsis in August of 2022 after legally taking abortion pills to end her pregnancy with twins. Her death, which was first reported by the left-leaning ProPublica, was quickly blamed on the state’s six-week abortion restriction without direct evidence, although a review committee found that there was a “good chance” earlier medical intervention could have prevented her death and pointed to the hospital’s “lack of policies/procedures in place to evaluate the uterus immediately.” The committee recommended all hospitals implement policies “to treat a septic abortion on an ongoing basis.”
Thurman’s sepsis death after a failed medication abortion was used by Democrat presidential candidate Kamala Harris during her campaign to slam now-President Donald Trump for his role in nominating the Supreme Court justices who ultimately overturned Roe.
Another medication abortion that allegedly ended in a serious adverse event is at the center of a legal battle between Texas and New York — as red states restrict medication and telehealth abortions and blue states pass shield laws which legally protect abortionists who send abortion pills into states with restrictions.
In December of 2024, Texas announced a lawsuit against a New York doctor for allegedly sending abortion pills to a woman in the state, which resulted in the death of an unborn child and serious complications for the mother. The complaint alleges that the 20-year-old woman asked to be taken to the hospital for “hemorrhage and severe bleeding” after taking the pills while nine weeks pregnant.
The lawsuit will be the first to test a battle between a pro-life state that restricts abortion pills and a pro-abortion state (New York) that passed a law legally shielding doctors who send abortion drugs into states that restrict abortions.
Three Republican-led states, Idaho, Missouri, and Kansas, this year were also given the clear to proceed with a lawsuit challenging the FDA’s deregulation of mifepristone. The states are challenging the FDA after the Supreme Court ruled in June of 2024 that the pro-life doctors who brought the original lawsuit lacked standing to sue. The high court ultimately did not rule on whether the FDA’s rollback on mifepristone regulations is legal.
At the same time, Trump’s Department of Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. indicated at his confirmation hearing in January that he is supportive of boosting transparency around mifepristone.
Sen. James Lankford (R-OK) asked Kennedy about his approach to mifepristone during the hearing and noted how the FDA stopped requiring the reporting of all complications related to abortion drugs in 2016, with the exception of deaths. Lankford also pointed out how the FDA under Biden allowed abortion pills to be prescribed over telehealth and sent via mail.
Lankford asked:
My question to you is, will FDA move to be able to actually give transparency to the American people and to say [mifepristone] is no different than any other drug? [That] we are not going to protect it just because [abortion] is political for some folks? People should know side effects of this drug and there should be reporting.
“It’s against everything we believe in this country that patients or doctors should not be reporting adverse events,” Kennedy replied. “We need to know what adverse events are. We need to understand the safety of every drug — mifepristone and every other drug. President Trump has made it clear to me that this is one of the things he has not taken a position yet on, a detailed position, but he’s made it clear to me that he wants me to look at safety issues, and I’ll ask NIH and FDA to do that.”
Last week, FDA Commissioner Martin Makary said he has “no plans to take action” to restrict the availability of mifepristone.
However, Makary did say there is a possibility the FDA could take action if data reveal dangers of the drug.
RELATED: Dashing, Darling! Women Dressed as Abortion Pills March Across Chicago
“There is an ongoing set of data that is coming into the FDA on mifepristone,” he said. “So if the data suggests something or tells us that there’s a real signal, we can’t promise we’re not going to act on that data.”
The study’s authors ultimately call on the FDA to reinstate the original patient safety protocols required when mifepristone was first approved and contends that “doing so will likely reduce the harms to women and permit better monitoring to determine whether this drug should remain on the market.”
“Our research shows unequivocally that mifepristone abortion, as currently practiced in the U.S., is considerably more dangerous to women than is represented on the FDA-approved drug label,” they wrote.
“The FDA should immediately reinstate its earlier, stronger patient safety protocols to ensure physician responsibility for women who take mifepristone under their care, as well as mandate full reporting of its side effects,” they continued. “The FDA should further investigate the harm this drug causes to women and, based on objective safety criteria, reconsider its approval altogether. Women deserve better than the abortion pill.”
The authors wrote that the paper is “the first in a series investigating women’s health and abortion using real-world data.”
Katherine Hamilton is a political reporter for Breitbart News. You can follow her on X @thekat_hamilton





Comments are closed.