Exclusive: See The ‘Airtight’ Methodology Showing 11% Of Pill Abortions Cause Maternal Injury
The most comprehensive U.S. study of the abortion pill excluded tens of thousands of insurance claims from its analysis of mifepristone-linked complications to ensure it did not exaggerate the harms the abortion drug could inflict on women.
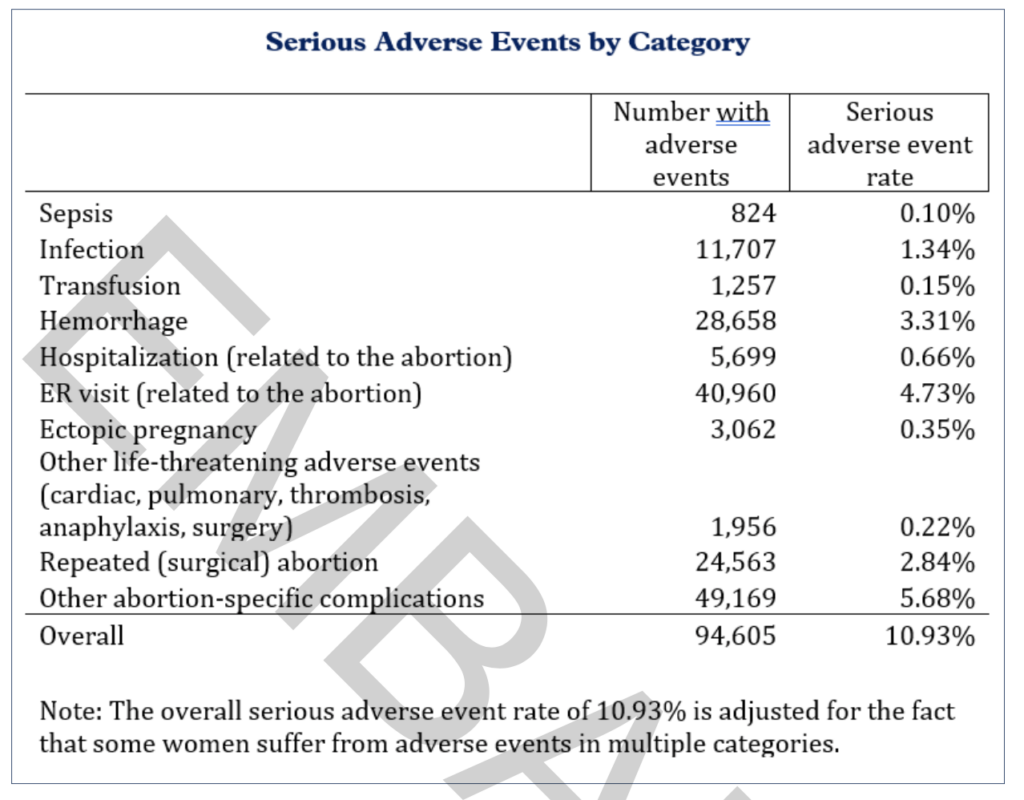
The exclusion of those data points further legitimizes findings that 10.9 percent of women suffered sepsis, infection, hemorrhaging, or other serious complications within 45 days of a chemical abortion. It also puts to bed criticisms raised by bad-faith actors who questioned researchers’ conclusions that the U.S. Food and Drug Administration should reinstate mifepristone safeguards or potentially rescind its approval altogether.
Corporate media, which have spent the years since the U.S. Supreme Court’s Dobbs v. Jackson decision aiding Democrats’ abortion for all agenda, largely ignored the landmark study. Those outlets that did mention it claimed the “bogus” findings were “junk science” that stemmed from “bad data.”
Methodology documents obtained by The Federalist, however, show 72 percent (or 98,483) of the emergency room visits represented across the Medicaid, TRICARE, Medicare, Department of Veterans Affairs, and private medical insurance claims purchased for the study were excluded because they were either unrelated to the abortion pill or failed to meet the medically serious threshold outlined by the National Institutes of Health.
Ethics and Public Policy Center researchers Ryan T. Anderson and Jamie Bryan Hall told The Federalist that they were careful to exclude certain ER diagnoses such as shortness of breath, nausea with vomiting, unspecified lower abdominal pain, Covid-19 exposure, nicotine dependence, asthma, and anxiety disorder to avoid overstating the risks of mifepristone.
Also kept out of the study’s ER visit data were the number of women who sought emergency treatment for miscarriage as opposed to women whose coded insurance claims indicated an elective chemical abortion and insurance claims from women who sought hospital help for what was determined to be “typical expected bleeding” after taking mifepristone.
“We intentionally did not include those because we weren’t trying to be sloppier to cook the books or to artificially inflate the number. We wanted this to be an airtight study, because we want the FDA to replicate it,” Anderson said.
The result was a 4.7 percent rate of abortion-related ER visits that involved serious adverse events, just one-tenth of a percentage point higher than the hospital visit frequency listed on Mifeprex’s label.
Even with tens of thousands of data exclusions and an ER visit benchmark that nearly matched the abortion drug’s label, Anderson and Hall found the rate of life-threatening complications due to mifepristone is at least 22 times higher than what the FDA and the abortion pill’s manufacturer suggest.
Replicability Is Key
At least one outlet complained that the lack of peer review on EPPC’s findings rendered it unscientific. Anderson and Hall said, however, that they opted to conduct a report that could be copied by the governing U.S. drug agency, skeptics, or even pro-abortion organizations like Planned Parenthood and Guttmacher Institute over the time-consuming peer review process because “the thing that matters most is replicability and peer review doesn’t even guarantee that.”
“The reality here is the peer review process is broken,” Anderson said. “This study would have taken years to get through a peer review process, because the editors would have slow-walked it.”
Even if Anderson and Hall submitted their findings to a journal to be evaluated and approved, the long-term publication of information exposing the dangers of the nation’s most popular abortion drug regimen is not guaranteed — as seen by the contested retractions of three peer-reviewed published studies on the harms of mifepristone shortly before the U.S. Supreme Court was slated to hear arguments in a landmark mifepristone case.
To further shore up their findings against criticism, Anderson and Hall ensured the seven years’ worth of insurance claims they used to evaluate mifepristone’s effects on women were a widely accepted, legitimate source used to inform academic research. In fact, President Donald Trump’s FDA commissioner, Martin Makary, has co-authored at least five studies that relied on de-identified patient data.
What Now?
In the conclusion of their study, Anderson and Hall warned that the FDA “should immediately reinstate its earlier, stronger patient safety protocols” and “reconsider its approval altogether.”
“We fully expect that if the FDA looks into this data, they’re going to come to the same conclusions,” Anderson confirmed to The Federalist.
Makary had hinted that new data could change the agency’s trajectory. The Federalist recently pressed Makary on evaluating mifepristone’s approval and safeguards and asked if he has plans to incorporate this study’s findings into his abortion pill decision-making. An FDA spokeswoman responded, claiming that “the agency rigorously evaluates the latest scientific data, leveraging gold standard science to make informed decisions.”
“I think it would be very hard to justify them simply putting their head in the sand and ignoring this. They promised to Make America Healthy Again, and you can’t Make America Healthy Again when 11 percent of women who have a chemical abortion suffer a serious adverse event,” Anderson concluded.
Jordan Boyd is a staff writer at The Federalist and producer of The Federalist Radio Hour. Her work has also been featured in The Daily Wire, Fox News, and RealClearPolitics. Jordan graduated from Baylor University where she majored in political science and minored in journalism. Follow her on X @jordanboydtx.





Comments are closed.